Molybdenum Phosphide Single Crystals for Efficient Hydrogen Peroxide Production
Sponsored by

Sponsored by

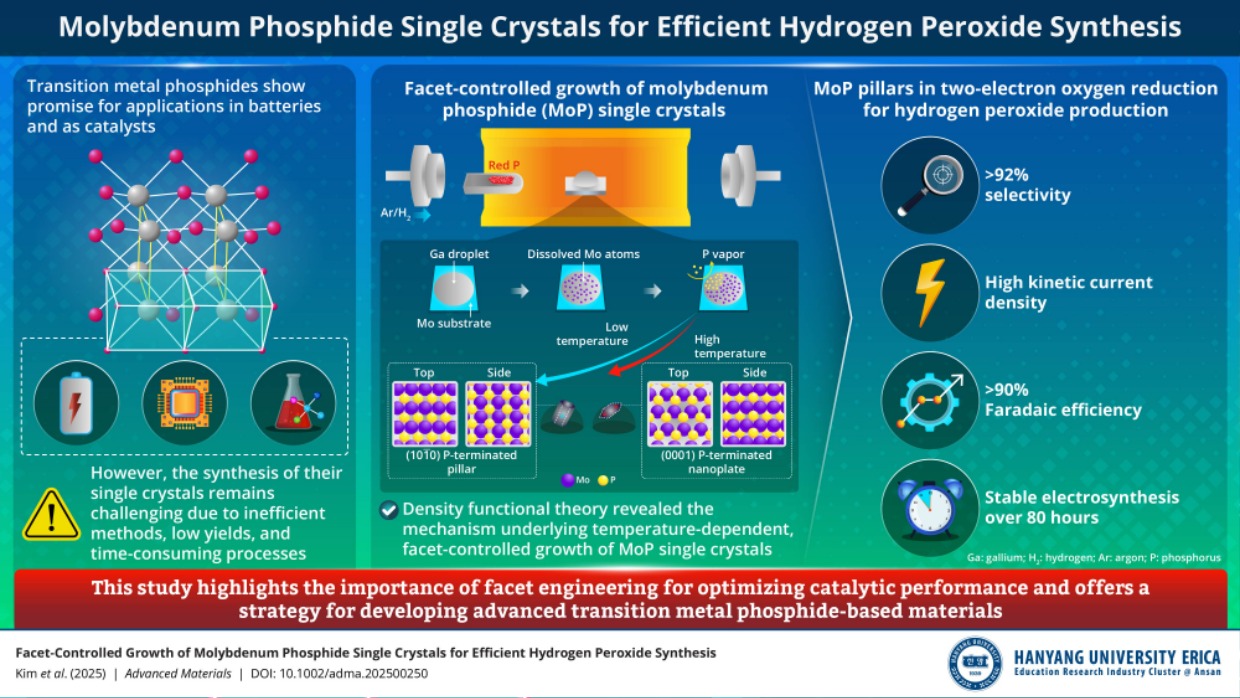
Transition metal phosphides are known to be promising materials for batteries and catalysts. However, the synthesis of their single crystals is difficult. Recently, researchers from Hanyang University ERICA have achieved the facet-controlled fabrication of molybdenum phosphide single crystals, furthering efficient hydrogen peroxide production. These findings are expected to make a ripple effect across various industries, especially in sustainable energy and environmental sectors.
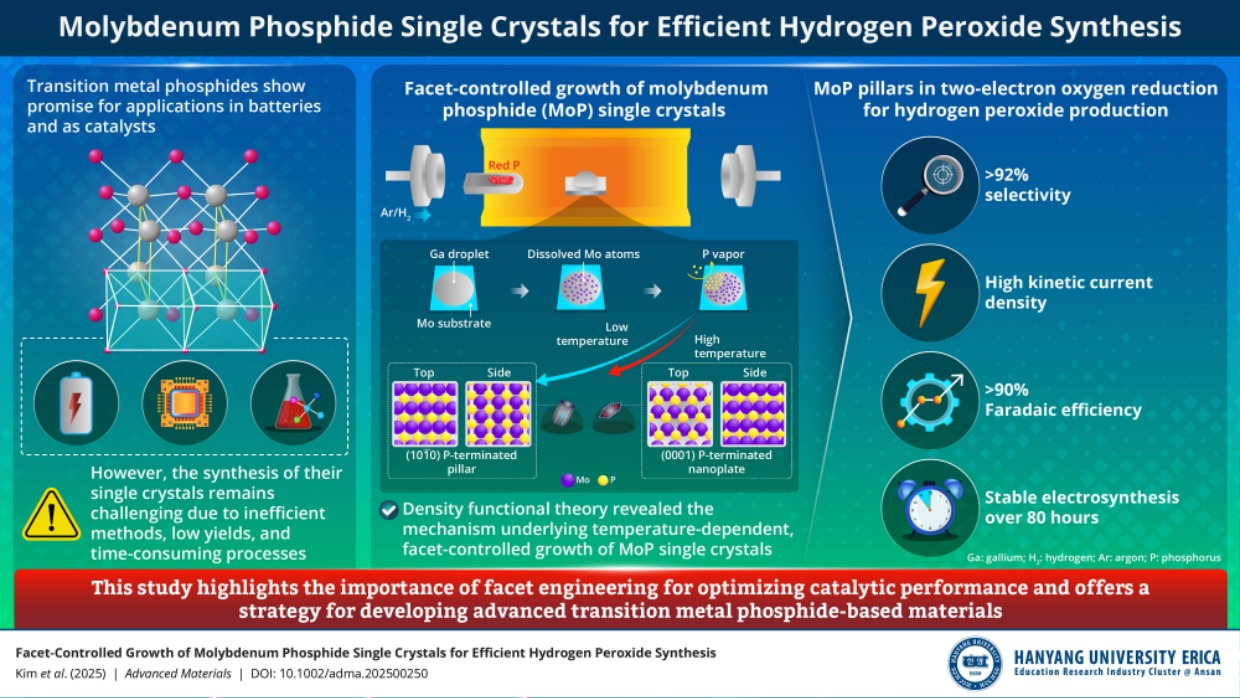
Image title: Molybdenum Phosphide Single Crystals for Efficient Hydrogen Peroxide Synthesis
Image caption: Researchers demonstrate that the pillar-shaped crystals are promising catalysts for efficient hydrogen peroxide production.
Image credit: Byung-Hyun Kim from Hanyang University ERICA
License type: Original Content
Usage restrictions: Cannot be reused without permission
Transition metal phosphides (TMPs) are an exciting class of materials. Due to the metal-phosphorus bonding that promotes electron donation, they possess outstanding catalytic properties for efficient electrocatalysis. However, fabricating single crystals of TMPs is challenging due to the lack of efficient techniques, poor yields, and time-consuming process steps.
In a recent breakthrough study, a team of researchers, led by Associate Professor Byung-Hyun Kim, Department of Energy and Bio Sciences at Hanyang University ERICA, achieved the synthesis of facet-controlled molybdenum phosphide (MoP) single crystals via a novel liquid-metal-assisted chemical vapor deposition method. Their findings have been made available online and published in Advanced Materialson 28 May 2025.
Dr. Kim says, “The primary objective of our study was to investigate how temperatureinfluences the material's morphology and how such temperature-induced morphological changes affect its oxygen reduction reaction, or ORR, characteristics using density functional theory (DFT) calculations."
Through DFT calculations, the team first identified the most stable crystal plane during crystal growth, predicting preferential growth initiation from this plane during synthesis. The calculations confirmed that the crystal's growth direction shifts from vertical to horizontal as the temperature rises. This morphological transformation mechanism is difficult to elucidate solely through experimental approaches, emphasizing the necessity of theoretical methods like DFT for precise atomic-level understanding. Furthermore, DFT calculations predicted that the pillar-shaped MoP would exhibit superior performance in the two-electron ORR compared to the nanoplate shape, a prediction verified by electrochemical experiments.
In this way, this work is significant because it identifies the mechanism behind temperature-driven morphological changes in MoP crystals using DFT and demonstrates that the pillar-shaped MoP is more effective and robust as an ORR catalyst. It achieves over 92% selectivity for hydrogen peroxide (H2O2) production as well asremarkably high kinetic current density.
The present study holds significant promise for applications in sustainable energy and environmental fields. “We demonstrated that MoP single crystals can serve as highly efficient catalysts for H2O2 production through precise control of crystal growth and morphology using temperature regulation and DFT calculations. Specifically, MoP crystals synthesized in a pillar form demonstrated exceptional catalytic performance, characterized by high selectivity and remarkable stability. Given the critical role of H2O2 as an oxidizing agent across various industrial processes, our approach is anticipated to enable more efficient and sustainable H2O2 production,” remarks Dr. Kim.
Within the next five to ten years, the computational approach presented in this study could enable the development of more efficient and environmentally friendly catalytic systems. Extending this methodology to a range of catalytic materials beyond MoP has the potential to significantly reduce environmental impact and operational costs. Furthermore, the insights from the present research can accelerate the development of advanced catalysts, creating positive ripple effects across diverse fields, including clean energy conversion, energy storage systems, and sustainable chemical synthesis. These technological advancements have the potential to significantly reduce environmental pollution, resource consumption, and greenhouse gas emissions, thereby actively promoting global sustainability.
Overall, this work is expected to pave the way towards the design of next-generation TMP-based materials for energy and sustainability applications.
Reference
Title of original paper:
Facet-Controlled Growth of Molybdenum Phosphide Single Crystals for Efficient Hydrogen Peroxide Synthesis
Journal:
Advanced Materials
DOI:
https://doi.org/10.1002/adma.202500250
About Hanyang University ERICA
Hanyang University ERICA (Education Research Industry Cluster at Ansan) is a prominent research-focused campus established in 1979 in Ansan, South Korea. ERICA offers undergraduate and graduate programs. ERICA is renowned for its active industry-university cooperation, offering students hands-on experience through partnerships with various industries. This ensures that graduates are well-prepared to meet societal needs and excel in their respective fields. With state-of-the-art facilities and a supportive learning environment, Hanyang University ERICA empowers students to pursue their passions and contribute meaningfully to society, staying true to the university's founding philosophy of "Love in Deed and Truth."
Website: https://www.hanyang.ac.kr/web/eng/erica-campus1
About the author
Professor Byung-Hyun Kim is an Associate Professor in the Department of Energy and Bio Sciences at Hanyang University ERICA. His group develops energy materials through multiscale simulations and artificial intelligence. Current research focuses include electrocatalysts for water electrolysis and fuel cells, advanced batteries, and semiconductors. Before this role, he served as a Principal Research Scientist at the Korea Institute of Energy Research and as a postdoctoral researcher in the Department of Chemistry-Ångström Laboratory at Uppsala University. He earned his Ph.D. in the Division of Materials Science and Engineering at Hanyang University.