LI-RADS for Diagnosing Hepatocellular Carcinoma in Patients with Noncirrhotic Chronic Hepatitis C
Addressing an important knowledge gap, researchers validate the diagnostic performance of LI-RADS – a widely used criteria in this high-risk population
Sponsored by

Sponsored by

Addressing an important knowledge gap, researchers validate the diagnostic performance of LI-RADS – a widely used criteria in this high-risk population
Liver Imaging Reporting and Data System (LI-RADS) category 5 (LR-5) is a well-established tool for diagnosing liver cancer from MRI and CT images but has not been validated in patients with chronic hepatitis C (CHC) without cirrhosis. Now, however, researchers from Korea tested the performance of LR-5 in this growing, high-risk population, finding it to be highly accurate, suggesting that noninvasive imaging could reliably diagnose liver cancer in noncirrhotic CHC patients.
Image title: Patients with noncirrhotic hepatitis C can benefit from noninvasive imaging diagnosis
Image caption: Researchers from Korea have found the LR-5 criteria to have excellent diagnostic accuracy and specificity. These findings suggest that LI-RADS system using CT and MRI scans can reliably diagnose hepatocellular carcinoma in patients with chronic hepatitis C who do not have liver cirrhosis, filling a critical gap in clinical guidelines.
Image credit: The authors
License type: Original Content
Usage restrictions: Cannot be reused without permission
Hepatocellular carcinoma (HCC), a common form of liver cancer, is a significant health concern for individuals with chronic hepatitis C (CHC). This risk persists even in patients who have not yet developed cirrhosis, a condition that represents advanced scarring of the liver, usually considered the highest risk factor for HCC. For other high-risk groups, such as those with cirrhosis or a chronic hepatitis B infection, HCC can be reliably diagnosed without a biopsy using characteristic imaging features from computed tomography (CT) and magnetic resonance imaging (MRI) scans. The Liver Imaging Reporting and Data System (LI-RADS) is the most widely adopted framework for this type of noninvasive diagnosis.
Unfortunately, however, LI-RADS has not been formally validated in patients with noncirrhotic CHC. This has real consequences for patient care, because liver biopsies carry risks like bleeding and may produce inconclusive results, especially for smaller tumors. Moreover, since new antiviral treatments can cure hepatitis C in over 95% of patients, there is now a growing population of cured individuals who still face elevated risk of developing liver cancer but fall outside the traditional high-risk categories for LI-RADS-based diagnosis.
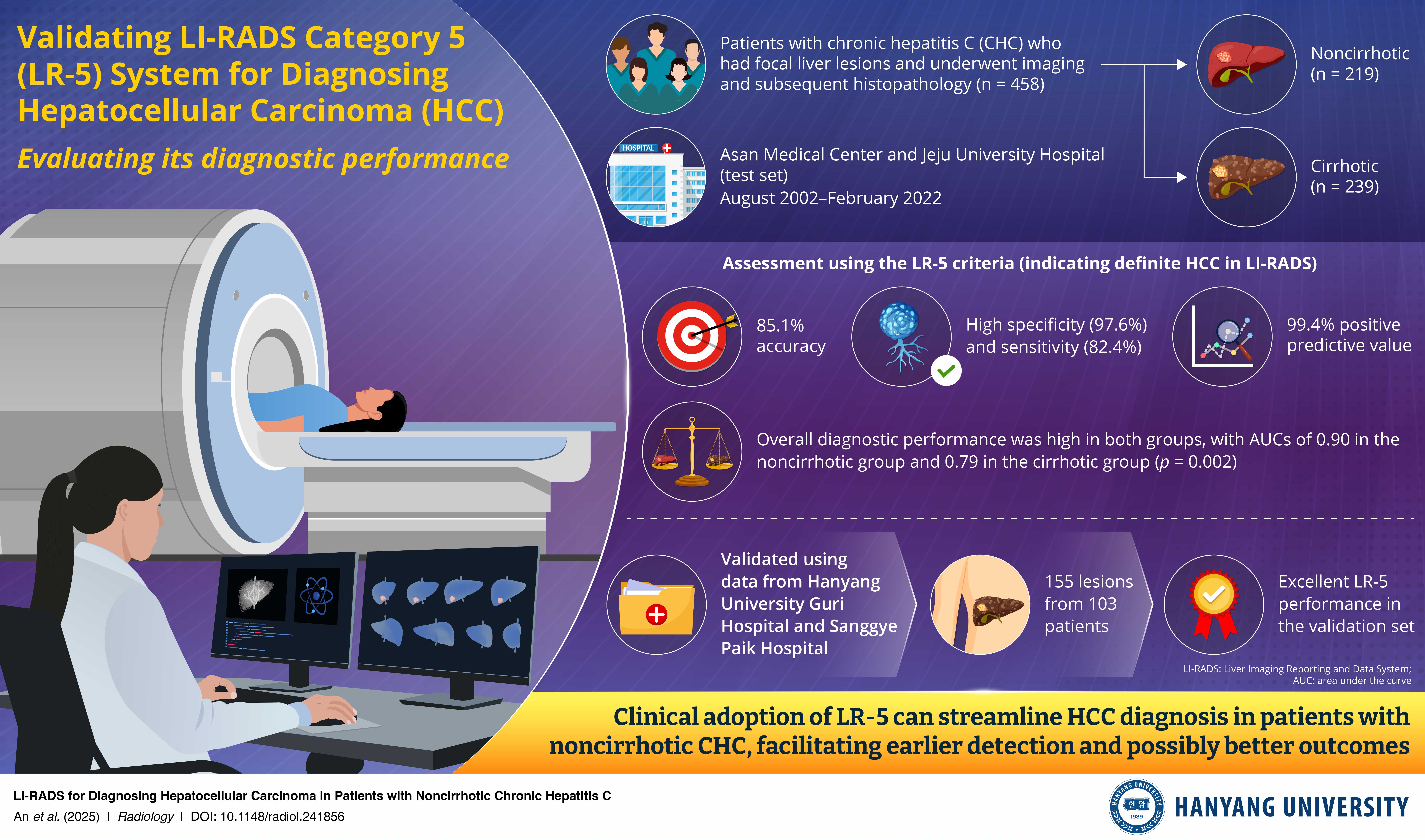
Now, a research team led by Dr. Jihyun An from the Department of Gastroenterology at Hanyang University College of Medicine, Korea, has addressed this knowledge gap. As explained in their paper, available online in Radiology on March 25, 2025 and published in the Volume 314, Issue 3 of the journal, they rigorously tested whether the LI-RADS category 5 (LR-5) criteria—which indicates definite liver cancer—could accurately diagnose HCC using CT and MRI scans in patients with CHC, comparing the results between patients with and without cirrhosis.
The study included a test dataset of 458 patients and a validation dataset of 103 patients with CHC, with data for each group gathered from different medical institutions. The LR-5 criteria demonstrated a high degree of accuracy for diagnosing HCC in noncirrhotic livers. In the test dataset, the LR-5 criteria achieved an accuracy of 85.1%, with a sensitivity of 82.4% and an excellent specificity of 97.6%. Notably, the diagnostic performance of LR-5 for patients with noncirrhotic CHC was comparable to its performance in patients with cirrhosis and was further confirmed in the validation dataset, maintaining a high diagnostic accuracy of 96.1%.
These findings represent a significant step forward in clinical practice, as they provide strong evidence to support the use of noninvasive imaging to diagnose HCC in patients with noncirrhotic CHC. This can allow doctors to confidently diagnose HCC and initiate treatment without the need for a biopsy. Worth noting, this research also holds promise for improving healthcare equity and access. “ Widespread adoption of validated imaging criteria like LR-5 may reduce disparities in access to diagnosis, particularly in settings where biopsy is unavailable or contraindicated, ” explains Dr. An.
Overall, these findings paves the way for establishing LR-5 as a reliable and accurate tool for diagnosing HCC in patients without cirrhosis. While the authors call for future prospective studies to confirm these findings, their research provides a robust foundation for updating clinical guidelines and improving diagnostic pathways for a large population of patients at risk of liver cancer. " In the next five to 10 years, as more patients are cured of hepatitis C through direct-acting antivirals, a growing number will fall outside traditional high-risk categories for HCC. This study paves the way for updating HCC screening and diagnostic guidelines to include noncirrhotic CHC patients—potentially leading to earlier detection and better outcomes ," concludes Dr. An optimistically.
Reference
Title of original paper:
LI-RADS for Diagnosing Hepatocellular Carcinoma in Patients with Noncirrhotic Chronic Hepatitis C
Journal:
Radiology
DOI:
About the author
Jihyun An is affiliated with the Department of Gastroenterology at Hanyang University College of Medicine, where she specializes in hepatocellular carcinoma and liver diseases. Her research primarily involves leveraging large-scale clinical data, meta-analyses, and genomic studies to discover new therapeutic targets and biomarkers for liver cancer. She is currently undertaking a visiting scholarship on liver cancer genomics at INSERM in France.
About Hanyang University
Hanyang University has pioneered higher education in Korea since 1939.
Rooted in the philosophy of 'Love in Deed and Truth,' we aim to cultivate global innovators.
Through cutting-edge R&D, international collaboration, and sustainable innovation,
Hanyang is positioning itself as a global hub for academic excellence and societal impact.