High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries
Researchers investigate the degradation factors of nickel-rich cathode active materials in all-solid-state batteries and identify their suppression methods.
Sponsored by

Sponsored by

Researchers investigate the degradation factors of nickel-rich cathode active materials in all-solid-state batteries and identify their suppression methods
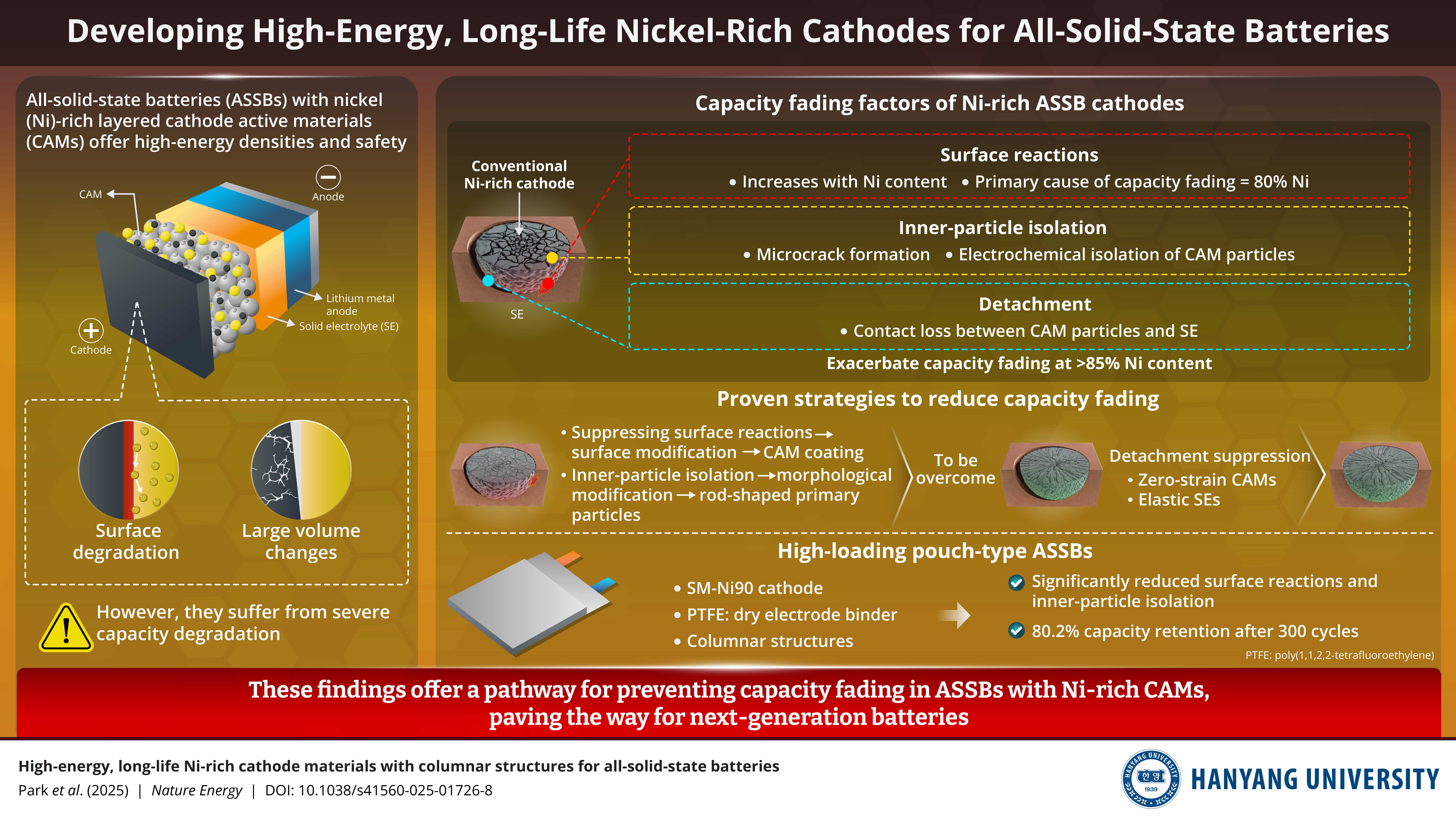
All-solid-state batteries (ASSBs) with nickel (Ni)-rich layered cathode active materials (CAMs) are promising alternatives to conventional lithium-ion batteries with flammable liquid electrolytes. However, integration of Ni-rich CAMs with solid electrolytes remains challenging, leading to severe capacity degradation. Now, researchers have systematically investigated the causes which lead to performance degradation of Ni-rich CAMs in ASSBs and identified methods to suppress them. This study serves as a foundation for developing next-generation, high capacity, and safe ASSBs.
Image title: Degradation factors of nickel-rich cathode active materials
Image caption: Appropriate surface and morphological modifications can suppress surface and inner-particle isolation reactions in nickel-rich cathode active materials. While suppressing detachment remains challenging, recent research points towards the use of zero-strain CAMs and elastic solid electrolytes as solutions.
Image credit: Professor Yang-Kook Sun from Hanyang University
License type: Original Content
Usage restrictions: Cannot be reused without permission
All-solid-state batteries (ASSBs) with non-flammable solid electrolytes (SEs) offer a much safer alternative. Yet, combining commonly used sulfide-rich SEs with Ni-rich CAMs remains challenging. At the CAM-SE interface, undesired surface reactions can degrade the cathode surface and reduce capacity. Ni-rich CAMs are also prone to microcrack formation, which interrupts physical contact and electrochemical reactions inside the secondary particles of the CAM, a phenomenon known as inner-particle isolation. In addition, volume changes during charge-discharge cycling can lead to detachment of the whole cathode particles from the SE. Together these issues accelerate the capacity fading of ASSBs.
To address these challenges, a research team led by Professor Yang-Kook Sun from the Department of Energy Engineering at Hanyang University, Korea, conducted the first systematic quantification of the key degradation factors in Ni-rich CAM degradation in ASSBs.“ The degradation mechanisms of Ni-rich CAMs in ASSBs with various factors have not been systematically investigated as of now, ” explains Prof. Sun. Adding further, he says, “ It is imperative to quantify each degradation factor of Ni-rich CAMs to develop high-performance ASSBs. In this study, we systematically analyzed these factors and identified methods to suppress them. ” Their study was published in Nature Energy on February 20, 2025.
The researchers synthesized four types of CAMs: pristine Ni-rich layered lithium-cobalt oxide CAMs (P-NCA), boron-coated CAMs (S-NCA), niobium (Nb)-doped CAMs (M-NCA), and both boron-coated and Nb-doped CAMs (SM-NCA). For each type, they prepared samples with Ni contents ranging from 80% to 95%.
The team identified three main factors for capacity degradation in Ni-rich CAMs: surface reactions, inner-particle isolation, and detachment. To isolate the effect of surface reactions, they compared S-NCA with P-NCA. The boron coating in S-NCA cathodes acts as a protective buffer between the CAM and SE, preventing surface reactions and enabling the researchers to measure degradation using this factor alone. Further, to evaluate inner-particle isolation, the team compared S-NCA and SM-NCA. Nb doping in M-NCA produces rod-shaped CAM particles, which suppress microcrack formation and therefore inner-particle isolation. Since SM-NCA also consists of boron coating, any remaining capacity loss in SM-NCA could be attributed mainly to detachment. Finally, to investigate the contribution of detachment, the capacity degradation of SM-NCA was compared to low-Ni CAM. This revealed that when the Ni content in CAMs exceeds 85%, capacity fading is dominated by both inner-particle isolation and detachment.
These findings demonstrate clear strategies for improving Ni-rich CAMs in ASSBs: surface reactions can be mitigated by surface modifications such as coatings, while inner-particle isolation can be suppressed by morphological modification of primary particles. Detachment, however, remains more difficult to control. As Prof. Sun remarks, “ Further research should be conducted to suppress detachment by developing zero-strain CAMs or elastic SEs. ”
To validate the benefits of surface and morphological modifications, the researchers fabricated a pouch-type ASSB, using an SM-NCA cathode with 90% Ni content. This cell retained an impressive 80.2% of its initial capacity after 300 cycles, achieving the highest specific capacity among previously reported ASSBs with Ni-rich cathodes. The outstanding performance was due to the cathode's ability to suppress surface reactions and its columnar structures, which prevented inner-particle isolation. The only remaining cause of capacity loss was detachment of the cathode from the solid electrolyte.
In summary, these findings not only establish a systematic framework for analyzing degradation in Ni-rich CAMs but also lay the groundwork for safer, high-performance ASSBs—paving the way toward a cleaner and more sustainable future.
Reference
Title of original paper:
High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries
Journal:
Nature Energy
DOI:
Prof. Yang-Kook Sun has been a Professor at Hanyang University, Korea, since 2000. He received his Ph.D. from Seoul National University. His research focuses on developing novel battery materials for a range of applications, including lithium-ion, sodium-ion, and all-solid-state batteries. One of his notable achievements is the commercialization of his innovative concentration gradient cathode materials, which have been used in major electric vehicles such as the Kia NIRO EV, Hyundai KONA EV, and Ford F-150 Lightning—since 2009.
About Dr. Nam-Yung Park
Dr. Nam-Yung Park is currently an Assistant Professor in the Department of Secondary Battery Convergence at Inha University in Korea. He received his Ph.D. in Energy Engineering from Hanyang University in 2024 under the supervision of Professor Yang-Kook Sun. His research focuses on cathode materials for lithium-ion and all-solid-state batteries.
About Hanyang University
Hanyang University has pioneered higher education in Korea since 1939.
Rooted in the philosophy of 'Love in Deed and Truth,' we aim to cultivate global innovators.
Through cutting-edge R&D, international collaboration, and sustainable innovation,
Hanyang is positioning itself as a global hub for academic excellence and societal impact.